Types of Polymer Matrix
- 5th July 2019
- Elliot Fleet
- Reading time: about 8 minutes

There are many different types of polymers that can be used as a matrix for composite materials. In this explainer, we’ll look at ways in which these materials can be categorised and highlight the key differences between them.
Let’s start with perhaps the most fundamental categorisation…
Thermoplastic or Thermosetting?
Thermoplastic
Thermoplastics can be heated and softened and then moulded or shaped. Upon cooling thermoplastics will hold their shape but can be softened and reshaped again upon heating. There are a huge range of thermoplastic polymers and they are typically supplied as solid pellets or powders. The ones most commonly used in composites are polypropylene and polyamides (nylons).

Thermoplastic composites are often favoured in industries where high production rate or recycling are important. The mechanical performance is usually lower than thermosetting composites, although high performance thermoplastics are available and gaining use in some specialised applications. However these high performance variants typically require very high processing temperatures, necessitating expensive processing equipment.
Notwithstanding the premium for high performance materials, the capital investments required in equipment and tooling are typically higher for thermoplastics than thermosets. For this reason thermoplastic materials are not ideal for short runs or prototypes (although 3D printers are attempting to address this problem). On the other hand when the production volumes increase, economies of scale tend to make the individual items relatively inexpensive.
Thermosetting
Thermosetting materials undergo an irreversible chemical reaction during moulding (known as crosslinking or cure) to go from a liquid or gummy state into the final state, which is generally solid. Once fully cured, these materials cannot be reshaped upon heating (to any significant extent). Thermosetting resins commonly used in composites include epoxies, unsaturated polyesters and phenolics.
 As thermoset materials generally require a period of time to cure, they are not as well suited to mass production as thermoplastics. However there are some resin systems and processes (for example RTM [link to explainer?]) that deliver reduced cycle times. Thermosets excel for short or medium runs in both low performance applications (e.g. traditional “fibreglass” applications) and high performance applications (e.g. aerospace, motorsport).
As thermoset materials generally require a period of time to cure, they are not as well suited to mass production as thermoplastics. However there are some resin systems and processes (for example RTM [link to explainer?]) that deliver reduced cycle times. Thermosets excel for short or medium runs in both low performance applications (e.g. traditional “fibreglass” applications) and high performance applications (e.g. aerospace, motorsport).
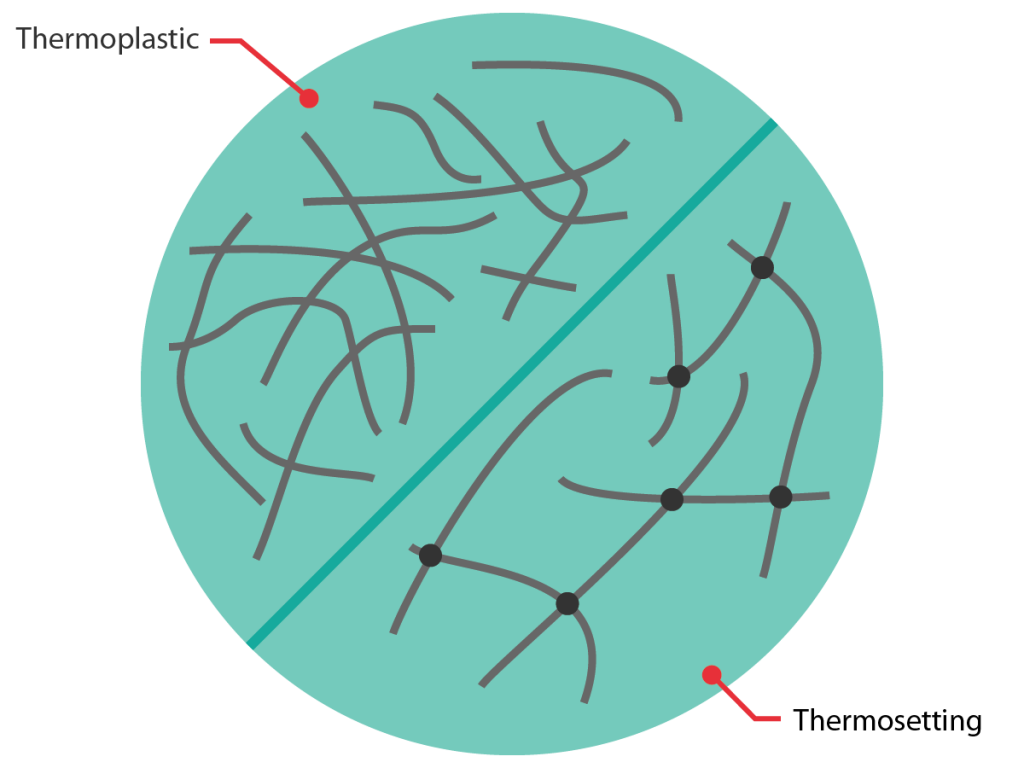
Thermosetting: Cross-links restrict the movement of polymer chains.
Crystalline or amorphous?
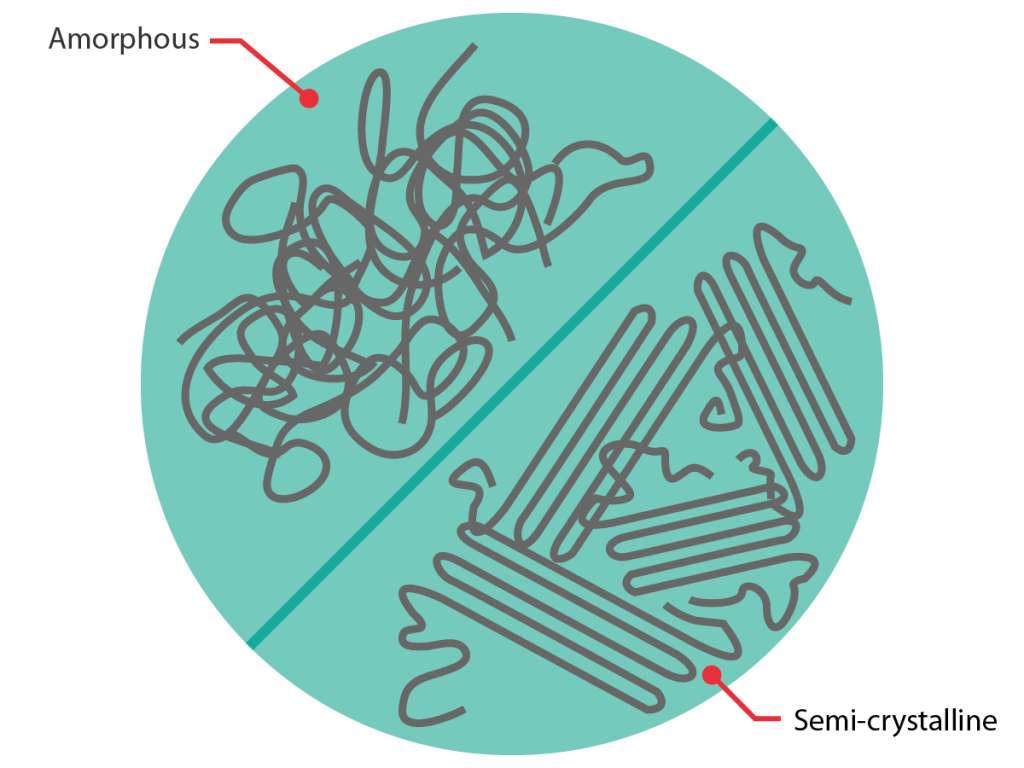
Materials can also be categorised according to their morphology: amorphous, crystalline or some way between the two extremes.
Crystalline materials feature atoms or molecules which are arranged in a regular, ordered way. Amorphous materials, on the other hand, lack this ordered structure; either because their molecules do not fit together, or because they have been cooled down so quickly that the molecules have not had time to arrange themselves in an ordered way. These differences in morphology lead to different behaviour upon heating.
For crystalline materials, the ordered molecules all gain mobility in a uniform manner as heat is applied. As a result, we observe a sharp transition from solid to liquid phase at a fixed melting temperature (Tm). Ice is a good example of this.
For amorphous materials, however, the disordered structure results in a non-uniform response to the application of heat. As a result, we observe a much more gradual softening over a wider temperature range.
The same categorisation can be applied to polymers with the exception that, although they can be purely amorphous (this applies to some thermoplastics and the vast majority of thermosets), polymers are never purely crystalline*. Instead they can be (and often are) semi-crystalline, meaning they feature a combination of crystalline and amorphous regions.
*Even the most crystalline examples (such as ultra-high molecular weight polyethylene) do not usually exceed 60% crystallinity.
A closer look at phase transitions in polymers
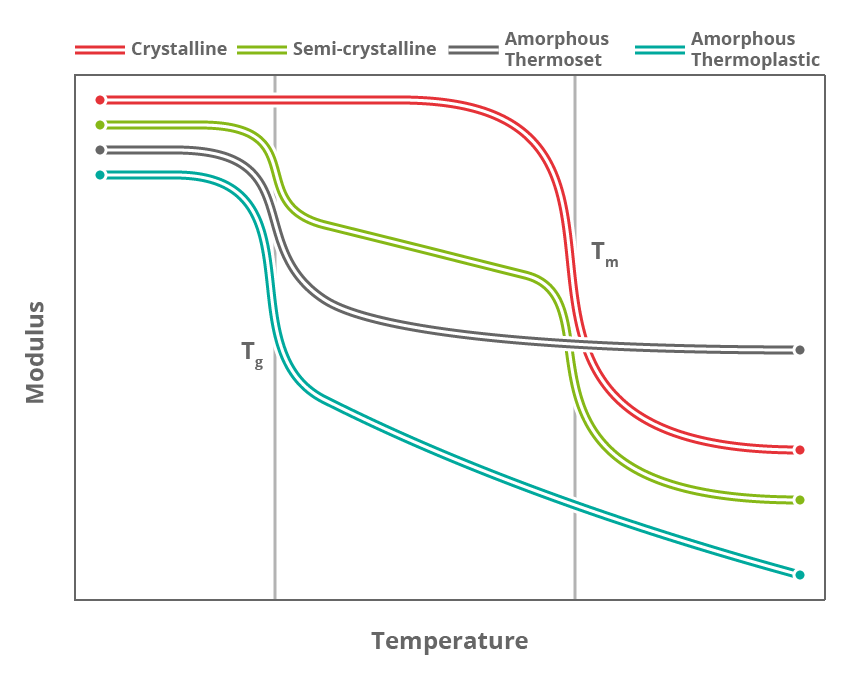
There are several different physical transitions that can happen to a polymeric material upon heating, but by far the two most important are melting and the glass-to-rubber transition (typically referred to simply as “glass transition”).
The melting point (Tm) is a temperature (or at least a narrow temperature window) in which crystalline sections start to move.
The glass transition (Tg) is a much more gradual change that is due to the “less organised” amorphous strings of molecules progressively gaining mobility. While the Tg is often reported as a single temperature this is actually a poor representation and it can vary by up to 50 °C depending on the chosen definition.
Classes of matrix
Semi-crystalline thermoplastics
Semi-crystalline materials feature both melting transitions and glass transitions. The dominant – and therefore most important – transition in semi-crystalline thermoplastics is the glass transition; above this point the polymer flows significantly and the material can be moulded.
If you heat a semi-crystalline thermoplastic above the Tg but below its melting temperature, you will generally find that toughness increases at the expense of stiffness; i.e. the polymer becomes more ductile. Polypropylene for example exists above its Tg at room temperature and is a relatively ductile polymer with low stiffness. If cooled below its Tg (~ -10°C) its stiffness will increase but it will also become a lot more brittle.
Common examples of semi-crystalline thermoplastics include the nylon family (polyamides). These materials are often used at room temperature (i.e. below their Tg of ~ 40 – 60°C) and offer high stiffness without compromising too much on toughness. Heating them above Tg will reduce the stiffness significantly but does also rapidly increase the impact strength. In composite applications, however, this decrease in nylon stiffness need not be an issue. This is because, typically, it is the reinforcement fibres (and not the matrix) which make the dominant contribution to the overall stiffness of the composite.
Amorphous thermoplastics
Amorphous thermoplastics do not feature a melting transition at all as they do not have any crystallinity. Materials such as these need to be heated to the top end of the Tg range (or above) in order to be moulded or formed.
In order for this class of materials to have useful engineering properties, the usage temperature has to be significantly below the glass transition range as there is no crystallinity present that can hold the material in shape. uPVC for example is an amorphous thermoplastic with a Tg commonly quoted as ~80°C which means it cannot usually be used above ~60°C.
Amorphous thermoplastics with a glass transition temperature below room temperature are not that useful but do sometimes get used as “tacky” adhesives.
Amorphous thermoplastics, with the exception of special materials such as ABS, are also usually optically transparent (at least before fillers are added). Semi-crystalline polymers however are universally opaque, as the crystals reflect light.
Some polymers are used in an amorphous form despite being capable of crystallisation. Polyethylene terephthalate (PET) for example is commonly used in carbonated drink bottles and is well-known to be more-or-less transparent (which means it cannot be crystalline). PET can, however, be a crystalline polymer. The reason it is amorphous in this application is due to the manufacturing method, it is cooled so fast that the crystallisation is not able to take place. If you take a PET bottle and heat it up above about 130°C it will start to become opaque as it crystallises.
Amorphous thermosets
The vast majority of thermosetting materials in use for composites applications are fully amorphous and therefore only feature a Tg. It follows, then, that Tg is the defining characteristic, which typically dictates the maximum service temperature of the final composite component.
Unless the cure process itself has given off excessive amounts of heat (which is usually undesirable) the glass transition temperature of cured amorphous thermosets is usually within about 20°C of the maximum cure temperature. This is why high temperature thermosets usually require a high temperature curing cycle.
Semi-crystalline thermosets
Melting transitions can also be measured within some types of thermosets such as semi-crystalline rubbers. However as the material is constrained by the cross-links, the melting transition represents nowhere near as dramatic a change as would be encountered in thermoplastics.
Phase transition in polymers
In summary
Matrix materials come in a variety of forms. To decide which one is right for your application , you will need to consider not only the performance requirements of a given component, but also the way in which that component will be manufactured. For more on this topic, try Coventive Explains: Can I have it in Composites?
Share this article
Found this article useful? We have a full range of services to help you...

Materials & Process Development
Whether it's thermosetting or thermoplastic composites, biocomposites or nanocomposites, we can help you develop a material or process that meets your requirements.
Developing composites...
Materials Characterisation & Testing
We have an extensive suite of testing facilities for characterising polymers and composites, coupled with the necessary expertise to interpret and advise upon test results.
Testing composites...About the author







